Abstract
Background: Intensive induction chemotherapy in the upfront setting of patients (pts.) with acute myeloid leukemia (AML) who do not harbour genetic alterations that confer chemosensitivity may not be satisfying. Likewise, the outcome of pts. with AML treated with intensive chemotherapy remains unsatisfactory in the relapsed/refractory (R/R) setting. FLAG-Ida (fludarabine, cytarabine and idarubicin) combined with venetoclax has recently been introduced as a novel therapeutic option for treatment of AML in the upfront and in the R/R setting in a series of early phase trials and 'real-world' clinical analyses. Early efficacy results are promising but there is still concern regarding the potential toxicity of this regimen. Therefore, we conducted a systematic review to assess the safety and efficacy outcomes of this treatment modality.
Methods: An electronic search for studies evaluating treatment combination of FLAG-Ida with venetoclax for pts. with AML was carried out. We included prospective trials and retrospective real-world studies. We excluded other intensive chemotherapy regimens. The primary safety outcomes were infections (bacterial and invasive fungal infections - IFI), time to neutrophil and platelets recovery and mortality within 30 days; the primary efficacy outcome was response to treatment (composite complete remission (CRc -as defined by each study) and overall response rate (ORR - per study definition)). Risk of bias was assessed according to the ROBINS-I tool.
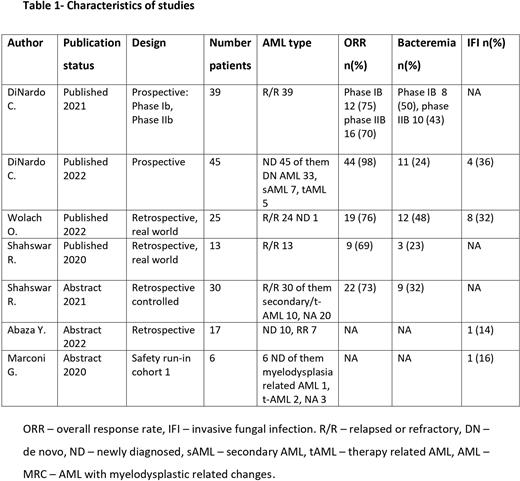
Results: A total of seven studies were included in our systematic review: five retrospective cohorts and two phase I/II studies. Four were published articles and three were abstracts. Both phase I/II studies were published by the same author (DiNardo et al.) with the latter being an extension of previously published data. Overall, 175 adult pts. with newly diagnosed (n=62) and R/R AML (n=113) were assessed (Table 1). Hematopoietic stem cell transplantation prior to treatment was reported in five studies (46/124 pts.). Four studies reported data on treatment with idarubicin (n=110/115 pts.), and three studies (101/114 of reported patients) elaborated on G-CSF treatment (during treatment, n=93 pts.; post treatment, n=8 pts). Five studies reported early death - overall total reported deaths 9/132 (6.8%), four pts. at 30 days, five pts. early death without time description. In particular 4/77 (5%) deaths were reported in the R/R group and 5/55 (9%) deaths in the newly-diagnosed (ND) group of pts.
Pooling of results was not conducted due to major differences between studies. Heterogenous safety and efficacy endpoints are presented among included studies, as well as heterogeneity in design.
Safety Outcomes: five studies reported neutropenic fever, ranging between 44-77%, five reported bacteremia with a range between 23-50%, four reported 12-30% pneumonia and four 4 reported 14-36% IFIs. Time to absolute neutrophil count (ANC) recovery and time to platelet recovery ranged between 23 and 33 days and between 31 and 35 days, respectively. Three studies presented time to count recovery of both ANC and platelets ranging between 21 and 37 days.
Efficacy outcomes: three studies reported CRc rates ranging between 61 to 89% for all pts. In the RR group, CRc rates ranged between 61 to 75% and CRc for ND (81%) was reported by one study only. In our cohort, five studies reported ORR, ranging between 69 to 98% for all pts. For the RR group ORR ranged between 69 to 76% and only one study reported an ORR for ND pts of 98%.
Risk of Bias analysis: When applying the ROBINS-I tool, six studies were judged to have serious risk of bias due to confounding based on inadequate management of possible confounders. Regarding possible bias in selection of patients, both phase II published studies were judged to have low risk of bias, one published retrospective study was judged to have moderate risk of bias and the remaining abstracts did not provide satisfactory data regarding methodology.
Conclusion: In our systematic review, FLAG-Ida plus venetoclax protocol proved to be a tolerable and effective regimen in newly diagnosed and R/R AML pts. Although infections may pose some concern, the overall toxicity is acceptable with promising response and remission rates for this challenging group of patients. We suggest to further evaluate and confirm the safety and efficacy of this new protocol in future RCTs.
Disclosures
Raanani:Janssen: Speakers Bureau; BMS: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Stahl:Boston Consulting: Consultancy; Novartis: Other: Advisory Board; Curis Oncology: Consultancy. Gafter-Gvili:Medison: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wolach:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jansen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.